INTRODUCTION
Methicillin-resistant Staphylococcus aureus (MRSA) is one of the leading causes of healthcare-associated infection, and it is implicated in infections of the bloodstream, lower respiratory tract, and the surgical site (Woolever et al., 2020). In 2017, the estimated cases in hospitalized patients were 323,700, and the estimated attributable health care costs were 1.7 billion dollars (CDC, 2019). Current guidelines from the Infectious Diseases Society of America (IDSA) and the American Thoracic Society (ATS) on community-acquired pneumonia (CAP) recommend empirically treating for MRSA in the following: patients who had previous isolation of Staphylococcus aureus from a respiratory culture in patients with CAP, and/or patients with severe CAP who had a history of hospitalization with parenteral antibiotics within 90 days (Metlay et al., 2019). However, empiric MRSA-targeted therapy is associated with increased medication costs and increases risk for antimicrobial resistance and adverse effects, including nephrotoxicity with vancomycin at rates noted from 5% to 43%, and thrombocytopenia with linezolid which has been reported to occur in around 3% to 5% of patients (Avdic et al., 2012; Cowley et al., 2019). Additionally, there is a decreasing prevalence of lower respiratory tract infections (LRTIs) caused by MRSA. A study of trends in antibiotic use in hospitalized veterans with pneumonia at 128 U.S. Department of Veterans Affairs medical centers noted an increase in vancomycin use from 16% in 2006 to 31% in 2010, despite a decrease in hospitalizations with cultures positive for MRSA from 2.5% to 2% (B. E. Jones et al., 2015; M. Jones et al., 2019). Therefore, identification of the causative pathogen in LRTIs is key in promoting judicious antibiotic use.
Utility of MRSA Nasal PCR Screening in Guiding Antibiotic Therapy for Bacterial LRTIs
Respiratory cultures are often used to determine the causative pathogen in hospitalized patients with bacterial LRTIs. These cultures may be retrieved from samples of expectorated sputum, endotracheal aspirate, or bronchoalveolar lavage (Baby et al., 2017). However, culture yield may be compromised by contamination with normal flora, receipt of antibiotics prior to collection, and the patient’s ability to produce adequate sputum samples (Marrie, 1994). Without objective microbiology data to guide de-escalation, MRSA-targeted antibiotics may be continued in patients for prolonged periods. Several MRSA screening tools exist and are performed in patients considered to be at high risk for colonization or infection. Samples are commonly taken from the anterior nares, and the estimated turnaround time for MRSA detection may be up to 24 hours (Rohrer et al., 2001).
The MRSA nasal swab polymerase chain reaction (PCR) assay can be used to rule out MRSA pneumonia with a negative predictive value (NPV) greater than 95% and a turnaround time of about one hour (Chou et al., 2018; Johnson et al., 2015). A single-center, retrospective study of patients with pneumonia was conducted at a community hospital (Chou et al., 2018). The study revealed that the NPV of the MRSA nasal swab for MRSA pneumonia was 0.97, while the positive predictive value (PPV) was 0.3 (Chou et al., 2018).
The high NPV of the MRSA nasal PCR for MRSA pneumonia drove clinicians to investigate its utilization in deprescribing strategies for pneumonia treatment (Dangerfield et al., 2014; Hiett et al., 2015). Several institutes adopted the policy of MRSA nasal PCR screening assays to guide MRSA antibiotics in pneumonia. A few studies have been published on the implementation of MRSA nasal PCR screening assay in antimicrobial use in pneumonia, but their outcomes varied (Baby et al., 2017; Diep et al., 2021; Woolever et al., 2020). Based on these findings, the decision was made to implement a pharmacist-driven protocol utilizing MRSA nasal PCR to facilitate the deprescription of MRSA-targeted antibiotics in pneumonia cases with negative PCR results.
Study Objective
The objectives of this study were to assess the impact of deprescribing MRSA-targeted antibiotics in pneumonia using MRSA nasal PCR screening assay on the duration of MRSA antibiotics and length of hospital stay. Understanding this impact highlights the potential for MRSA nasal PCR screening to optimize antibiotic stewardship, reduce unnecessary antibiotic exposure, and reduce hospital stay in hospitalized pneumonia cases.
METHODS
Ethical Approval
Written approval was obtained from the head of the Adventist Health Glendale Internal Review Board, Glendale, CA, USA (September 2019), and the project was exempted from complete ethics approval from the Adventist Health Glendale as the project was regarded as a quality improvement project to measure the impact of an implemented antimicrobial stewardship strategy to deprescribing antibiotics.
Study Design and Patient Population
This was a time series, quasi-experimental study conducted at an acute care community hospital. Patients in the pre-protocol group admitted prior to protocol implementation from October 1, 2018, to March 31, 2019, with an MRSA nasal PCR result were identified through the hospital’s clinical microbiology laboratory database. Based on the results of Chou et al (2018), the protocol for the present study was developed and approved in the community hospital’s Pharmacy and Therapeutic Committee. The study described in Chou et al (2018) was conducted in the same community hospital. The protocol detailed the utilization of negative MRSA nasal PCR results to de-escalate MRSA therapy in pneumonia patients. Patients who were admitted from October 1, 2019 (the date of protocol implementation) to March 31, 2020, were included in the post-protocol group.
Inclusion and Exclusion Criteria
Patients were in both groups were included if they were at least 18 years of age and received new medication orders for either IV vancomycin or IV/PO linezolid with the diagnosis of pneumonia as specified by the ordering provider.
Patients were excluded if they received a positive influenza PCR test result, a positive MRSA nasal PCR, or a positive MRSA sputum culture at any time during their hospital stay. Additional exclusion criteria were receipt of MRSA-targeted antibiotics within 30 days prior to their hospital stay, or receipt of MRSA decolonization agents such as intranasal mupirocin, bacitracin, or povidone-iodine prior to MRSA nasal PCR sample collection. The decision to exclude patients with a positive influenza PCR test result was based on literature discussing the association between the suppression of innate immunity and consequent defects in bacterial control after influenza infection (Metzger, 2013). An additional exclusion criterion for the post-protocol group was the presence of confirmed or suspected COVID-19. This criterion was added given the lack of information supporting the de-escalation of empiric MRSA antibiotics in patients with the disease at the time. Patients with confirmed COVID-19 had a positive SARS-CoV-2 PCR test result during hospitalization. Patients with suspected COVID-19 were defined as those who did not have a finalized SARS-CoV-2 PCR test at the time of investigation within the study period but were suspected of having the disease as noted in the medical team’s progress notes.
Protocol for MRSA Nasal PCR Screening and Antibiotic Deprescribing
Upon receiving computerized provider order for IV vancomycin or IV/PO linezolid with a pneumonia indication, the staff pharmacist ordered an MRSA nasal PCR per protocol. A new PCR screen was not ordered when an MRSA nasal PCR result within 7 days was available in the patient’s EMR. MRSA nasal PCR samples were collected by nurses and analysed using the GeneXpert MRSA assay (Cepheid, Sunnyvale, CA) at the hospital’s clinical microbiology laboratory. Antimicrobial stewardship pharmacists then followed up with the test result. When the result was negative, the pharmacist assessed the appropriateness for deprescribing MRSA antibiotics. When deemed appropriate, the pharmacist initiated an antibiotic deprescribing attempt with the provider based on the negative MRSA nasal PCR result. Methods of communication included phone calls, pages, and verbal discussions during rounds. Physicians were generally receptive to these pharmacist recommendations.
Measures
The following data were extracted retrospectively from the patient’s electronic medical record (EMR): age, gender, admission date and time, discharge date and time, date and result of MRSA nasal PCR collection, and dosing history of IV vancomycin or IV/PO linezolid. If available, the date and result of sputum culture were also collected.
The primary outcome was the duration of therapy for MRSA antibiotics. The duration of therapy was determined by totalling the number of days on IV vancomycin or IV/PO linezolid. For example, patients who received a total of one dose of the antibiotic before it was discontinued, were counted as having a duration of therapy of one day. The secondary outcome was the length of hospital stay, determined from the admission and discharge date and time in the patient’s EMR.
Statistical Analysis
Baseline demographic data including patient sex, presence of co-infections (defined as infections other than pneumonia, confirmed via the medical team’s progress notes in the patient’s EMR), presence of gram-negative organisms, and availability of sputum cultures were compared between the pre-protocol group and the post-protocol group using Chi-square test or Fisher’s exact test. For the duration of therapy, length of stay, and patient age, the Normality and Lognormality tests followed by the Mann-Whitney test were performed. A p-value of less than 0.05 was considered statistically significant. The contingency (sensitivity, specificity, positive predictive values, and negative predictive values) of nasal MRSA PCR screening and MRSA respiratory cultures was analyzed for the patients with nasal PCR and respiratory cultures. All the statistical analyses were performed by using GraphPad Prism version 9.3.1 for Windows, GraphPad Software, San Diego, California USA, wwwgraphpad.com.
RESULTS
Final Sample
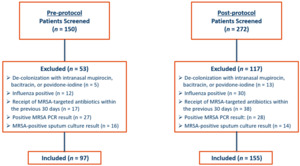
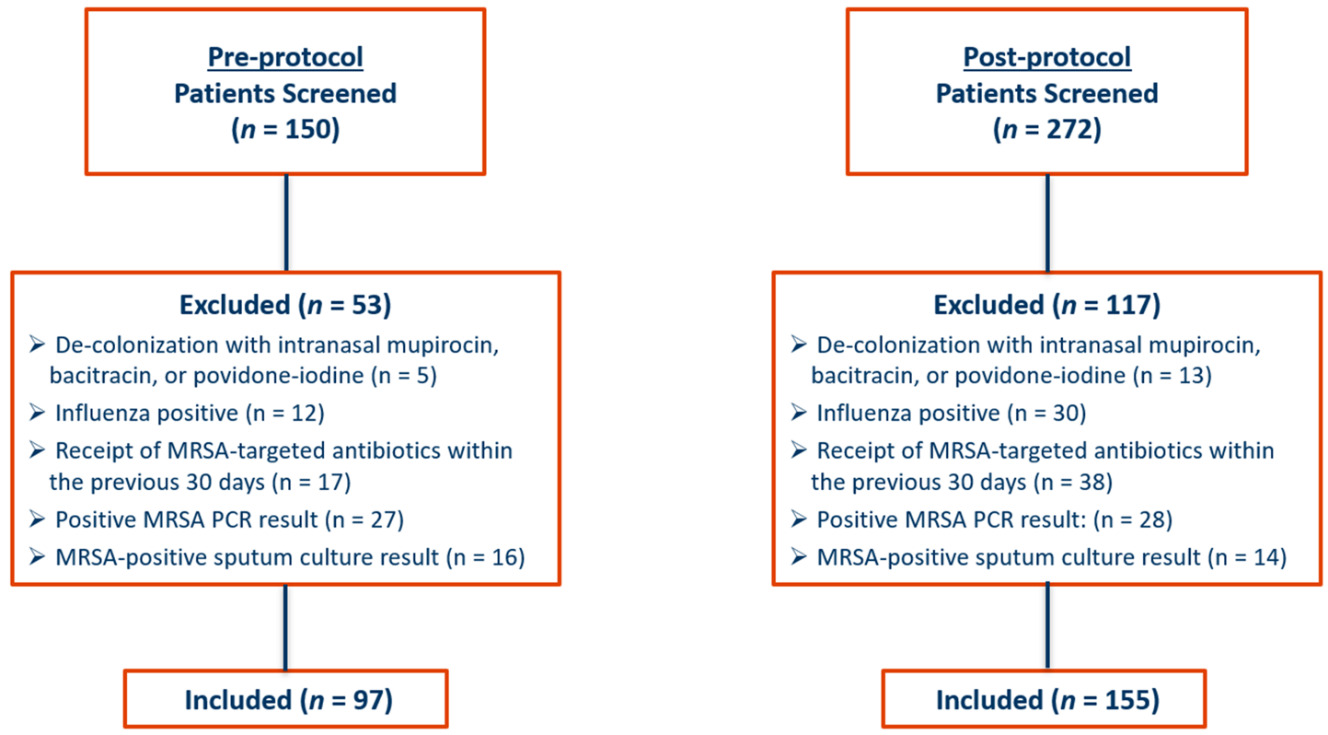
A total of 422 patients were screened. Ninety-seven patients admitted from October 1, 2018, through March 31, 2019, were included in the pre-protocol group, and 155 patients admitted from October 1, 2019, through March 31, 2020, were included in the post-protocol group. The most common reason for patient exclusion in the pre-protocol group was the presence of a positive MRSA nasal PCR result during hospital stay (n = 27). In the post-protocol group, most patients who were excluded from the final analysis received MRSA antibiotics within 30 days prior to admission (n = 38). Details for patient inclusion and exclusion are provided in Figure 1.
Comparison of Pre-protocol and Post-protocol Patients
Baseline characteristics are summarised in Table 1 and were similar in both groups regarding age, sex, presence of co-infections, and presence of gram-negative organisms in the sputum culture. In the pre-protocol group, the median age was 76 years, and in the post-protocol group, the median age was 75 years. Co-infections were present in 20 patients (21%) in the pre-protocol group, and in 37 patients (24%) in the post-protocol group (p = 0.548). Among these, urinary tract infections were the most common, comprising 11 co-infections (55%) in the pre-protocol group, and 18 co-infections (49%) in the post-protocol group. Co-infections included skin and soft tissue infection, bacteremia, urinary tract infection, osteomyelitis, intra-abdominal infection, surgical site infection, cholecystitis, and diabetic foot infection.
Gram-negative organisms were isolated in the sputum cultures of 10 patients (10%) in the pre-protocol group, and 12 patients (8%) in the post-protocol group (p = 0.48). Organisms included at least one of the following: Pseudomonas aeruginosa, Pseudomonas fluorescens, Enterobacter spp., Serratia spp., Escherichia coli, Proteus spp., Klebsiella pneumoniae, Morganella morganii, and Stenotrophomonas maltophilia. Non-MRSA gram-positive organisms isolated from sputum cultures included Group C Streptococcus, Group G Streptococcus, and methicillin-susceptible Staphylococcus aureus. The availability of sputum culture results differed between both groups, with 74 patients (76%) in the pre-protocol group having at least one available sputum culture result, and 78 patients (50%) in the post-protocol group having at least one available sputum culture result (p < 0.001).
Outcomes by Group
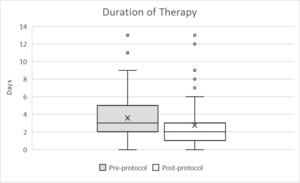
The median duration of empiric MRSA antibiotics in the pre-protocol group was 3 days (Interquartile range, IQR 2-5), and the median duration in the post-protocol group was 2 days (IQR 1-3), reflecting a decrease in the median duration of empiric MRSA antibiotics after protocol implementation by one day (p < 0.001). The median length of hospital stay in the pre-protocol group was 9 days (IQR 5-17) versus 7 days (IQR 5-13) in the post-protocol group. The post-protocol implementation was associated with a decrease in the median hospital length of stay by 2 days (p = 0.040). Further details regarding primary and secondary outcomes are summarized in Table 2 and Figure 2.
DISCUSSION
IDSA/ATS hospital-acquired and ventilator-associated pneumonia guidelines published in 2016 had not provided guidance on deprescribing MRSA antibiotics by using negative MRSA nasal swab screening. However, the IDSA/ATS community-acquired pneumonia guidelines in 2019 recommend obtaining cultures or nasal PCR to allow for de-escalation or confirmation of the need for continued MRSA therapy (Kalil et al., 2016; Metlay et al., 2019). This study found that implementation of a protocol utilizing a negative MRSA nasal PCR result was associated with a decrease in both duration of empiric MRSA antibiotics for pneumonia and length of hospital stay by 1 day and 2 days, respectively.
The study findings regarding the duration of therapy are consistent with those published in similar studies that assessed the impact of a pharmacist-driven protocol regarding de-escalating empiric MRSA antibiotics in pneumonia following a negative MRSA nasal PCR result. In those studies, the duration of empiric MRSA antibiotics was reduced by 10 to 47 hours after the protocol implementation with no significant difference in length of stay (Dadzie et al., 2019; Diep et al., 2021). The reduction in the hospital length of stay after protocol implementation in this study is consistent with the findings from Dadzie et al. (2019) and Willis et al. (2017). Cowley et al. (2019) also found that patients with de-escalation had significantly shorter hospital and/or ICU stay with no difference in 28-day mortality. This study results also confirms the decreased duration of therapy and shortened hospital length of stay.
Circumstances that may have led to an extended duration of therapy of MRSA antibiotics include the presence of co-infections, presence of sepsis or severe community-acquired pneumonia, variability or delay of MRSA nasal PCR sample collection, and lack of methicillin-susceptible Staphylococcus aureus antibiotics with a beta-lactam antibiotic due to severe penicillin allergy. Although the post-protocol group had significantly fewer sputum cultures compared to the pre-protocol group, this difference is unlikely to have influenced the duration of MRSA-targeted antibiotic therapy due to the high negative predictive value of the MRSA PCR test. The study focused solely on MRSA nasal PCR collection, with no data gathered on MRSA nasal screen cultures. MRSA nasal PCR has been reported to have equal or higher sensitivity to the MRSA nasal culture, and its turn-around time is much faster than MRSA nasal culture (Shenoy et al., 2014). Therefore, MRSA nasal PCR is more suitable to be used for deprescribing MRSA antibiotics in patients with negative results than MRSA nasal culture (Baby et al., 2017).
Limitations
There are several limitations to the study. The single-centered, non-matching, and non-randomized nature decreases external validity. The duration of empiric MRSA antibiotic therapy was measured in days rather than hours. This is consistent with reports from hospital antimicrobial stewardship programs which commonly express trends in hospital antibiotic usage in days of therapy. Due to this, it is possible that the duration of therapy may have been overreported or underreported for some patients compared to other studies. The protocol may also have an effect on additional meaningful clinical outcomes such as acute kidney injury incidence, thrombocytopenia incidence, 30-day hospital readmission rate, and 30-day mortality rate, although these have not been assessed in this study.
Further studies expanding upon the impact of de-escalating empiric MRSA antibiotics in infections other than pneumonia using MRSA nasal PCR are warranted. A study by Gentges et al. (2023) reported a significant decrease in vancomycin usage days of 2.34% per month over a two-year period after implementation of a hospital-wide intervention designed to increase the use and knowledge of MRSA nasal PCR screening. Patients included in the study were suspected of having potential for MRSA infections including pneumonia, intra-abdominal infections, bacteremia, and skin infections (Gentges et al., 2023). Stratification of patients into those with severe community-acquired pneumonia and those admitted to the ICU may provide useful insight into the impact of the protocol on these patient subpopulations. Assessment of pharmacoeconomic outcomes including direct drug costs and indirect drug costs such as therapeutic drug monitoring (TDM) of vancomycin and total hospitalization cost may be useful for evaluating the protocol’s impact on resource utilization.
CONCLUSIONS
This research study provides significant evidence for an effective strategy to de-escalate empiric antimicrobial agents in patients with lower respiratory tract infections. Implementation of a pharmacist-driven protocol to de-escalate MRSA-targeted antibiotics for pneumonia by utilizing MRSA nasal PCR was associated with a decrease in both the duration of empiric MRSA antibiotic therapy and the length of hospital stay. These findings make a remarkable contribution to antimicrobial stewardship strategies in de-escalating empiric MRSA antibiotics in pneumonia patients with negative MRSA nasal PCR results. Data from future randomized clinical trials would provide better evidence to use a negative MRSA nasal PCR result to withhold empiric MRSA therapy and shorten the duration of MRSA antibiotics in pneumonia patients.